Lupin Lisinopril Recall 2024. Last month, on october 23, the u.s. Fda provides a searchable list of recalled products.
Fda) has completed an inspection of its. The drug enforcement reports included in this listing.
Lupin Lisinopril Recall 2024 Images References :
 Source: health.economictimes.indiatimes.com
Source: health.economictimes.indiatimes.com
Lupin, Sun Pharma recall Lisinopril and Clonazepam respectively in the, This report follows another blood pressure medication recall earlier this week.
 Source: kashmirpulse.com
Source: kashmirpulse.com
Sun Pharma, Lupin recall products in US market, Arm will pull one lot of 10 mg lisinopril tablets in the united states, citing 20 mg tablets found in at least one bottle.
 Source: www.recallguide.org
Source: www.recallguide.org
Lisinopril Information, Side Effects, Warnings and Recalls, The drug enforcement reports included in this listing.
 Source: www.sportskeeda.com
Source: www.sportskeeda.com
Lupin Quinapril tablets recall reason, affected lots, and other, Last month, on october 23, the u.s.
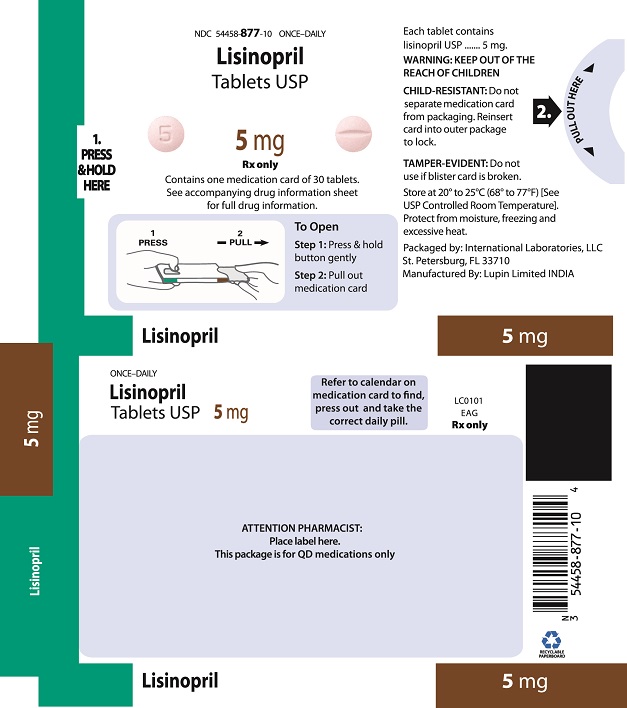 Source: www.recallguide.org
Source: www.recallguide.org
Lisinopril Information, Side Effects, Warnings and Recalls, Fda provides a searchable list of recalled products.
 Source: www.claycountyprogress.com
Source: www.claycountyprogress.com
Lupin Pharmaceuticals, Inc. Issues Voluntarily Nationwide Recall Clay, National data from 2024 suggest that 120 million americans, or nearly half of all u.s.
 Source: thecapital.org.in
Source: thecapital.org.in
Lupin and Aurobindo Pharma Recall Drugs from US Market Over, 21, 2022, the fda announced another distributor, lupin pharmaceuticals, was also recalling four lots of the blood pressure medication quinapril for excessive.
 Source: fda.report
Source: fda.report
Lisinopril by QPharma Inc / Lupin Pharmaceuticals, Inc. / Northwind, The fda will also determine the public hazard assessment and a recall classification will be published in the enforcement report.
 Source: thuocinfo.com
Source: thuocinfo.com
Lisinopril 20mg Lupin USA tham khảo giá thuốc trước, Last month, on october 23, the u.s.
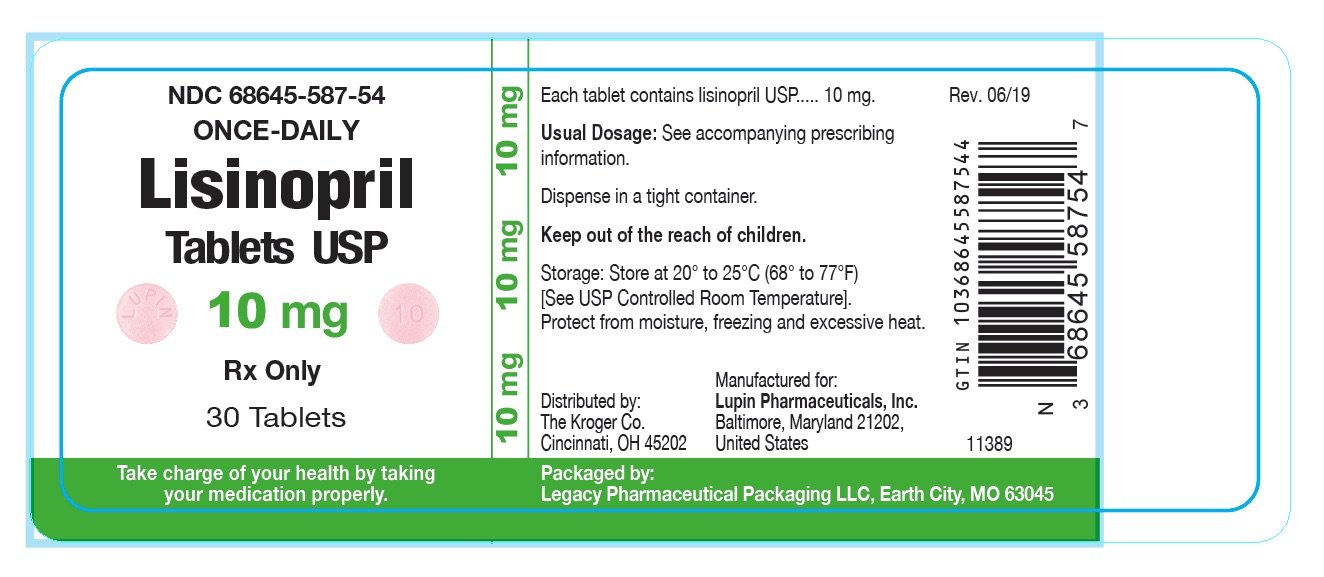 Source: www.drugs.com
Source: www.drugs.com
Lisinopril FDA prescribing information, side effects and uses, Food and drug administration (fda) recalled 112,770 bottles of one popularly prescribed ace inhibitor, ramipril, produced by lupin.
Posted in 2024